Methylation: Overlooked & Underappreciated
News and Science Behind Formulas • Cited Research • Discounts and Offers
The Human Genome Project, which began in 1990 and was completed in 2003, set out to map the entire DNA blueprint of the human species in hopes that they could reverse disease with the limited view that non-infectious illness came from a gene that could be corrected or medicated. The associated idea of genomic medicine failed to address the whole individual and outside factors that affect health, propelling the idea of epigenetics as a significant causal factor in disease development, severity and progression. Still today, the search for a genetic factor is heavily considered for unknown illness and consequently, the plethora of other factors that can cause unfamiliar symptomatology are grossly ignored.
Epigenetics and Methylation
To understand methylation, we must first come to understand epigenetics.
- Epigenetics began as an attempt to understand the process of a fertilized, single-celled ovum transforming into a complex, orchestrated organism. Over time, our understanding of the process has modified our definition of epigenetics to look at the expression of DNA, not based on structural changes of the DNA sequence, but rather the alteration of genetic expression through both chemical modifications occurring on DNA and the associated proteins that act upon DNA. This heritable expression that can be passed from mother cell to daughter cell, influenced by both internal and external factors, has recently become a main focus in personalized medicine.¹
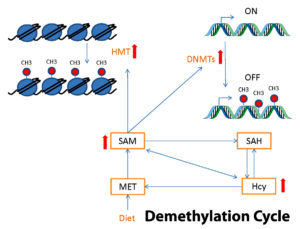
- Methylation, a heavily involved component of epigenetics, is a fairly simple process in which a methyl group, CH3 (one carbon and three hydrogens) attaches to a strand of DNA, but yet has vast implications on human physiology. This biochemical reaction either attracts proteins to silence genes, or prevents the attachment of transcription factors to genetic sequences that would keep genes “turned on.” Methylation and demethylation are constantly happening throughout every cell in the body to express and repress genetic information for the purpose of maintaining homeostasis in the body.
Methylation is responsible for gene regulation and is also involved in cell differentiation – the process of which developing cells become more specialized.² Methylation is highly concentrated in the central nervous system affecting brain/neurological function and myelination of nerves. It is also involved in cardiac function³ ⁴, immunity⁵, detoxification⁶, neurotransmitter production and metabolism,⁷ and metabolic and mitochondrial function⁸. Another area gaining recent interest is the effects of methylation on viruses and the reemergence of latent viruses that lie dormant within the host.⁹ ¹º
HEALTH DISORDERS ASSOCIATED WITH POOR METHYLATION ²³
| Diabetes | Downs Syndrome | Alzheimer’s/Dementia |
| Cancer | Autism | Schizophrenia |
| Fibromyalgia/CFS | Neuropathy | Anxiety |
| Pulmonary Embolism | Lyme Disease | Spina Bifida |
| Alcoholism | Chronic Infections | Neural Tube Defects |
| Addiction | Atherosclerosis | Cleft Palate |
| Insomnia | Autoimmune Disorders | Tongue/Lip Tie |
| Bipolar/Manic Depression | ADD/ADHD | Multiple Food Sensitivities |
DNMTs: Enzymes for Methylation
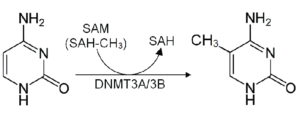
The action of methylation requires the enzymes DNA methyltransferases (DNMTs) to transfer the methyl group from S-adenosylmethionine (SAMe) to the DNA strand. These DNMTs, (DNMT1, DNMT3a, DNMT3b, DNMT3L) serve different functions in the methylation process.
As the DNA strand splits during replication, DNMT1 methylates the newly exposed strands to replicate an exact copy of the original strand. The repair of DNA methylation can also be addressed by DNMT1. DNMT3a and DNMT3b both have the ability to methylate naked DNA and differ by location of activity in tissues as well as activity in life stage. DNMT3a is mainly involved in normal cell differentiation, whereas NMT3b is critical for embryonic and early development. DNMT3L assists with the methylation of 3a and 3b and is only present in embryonic development and in germ cells of the thymus gland in adults.²
DNA strands wrap around proteins called histones to help package long strands into smaller compartments within the nucleus. DNMTs also work in conjunction with histone-modifying enzymes to tighten the DNA strands around the protein to prevent transcription of the genetic sequence.²
DNA strands wrap around proteins called histones to help package long strands into smaller compartments within the nucleus. DNMTs also work in conjunction with histone-modifying enzymes to tighten the DNA strands around the protein to prevent transcription of the genetic sequence.²
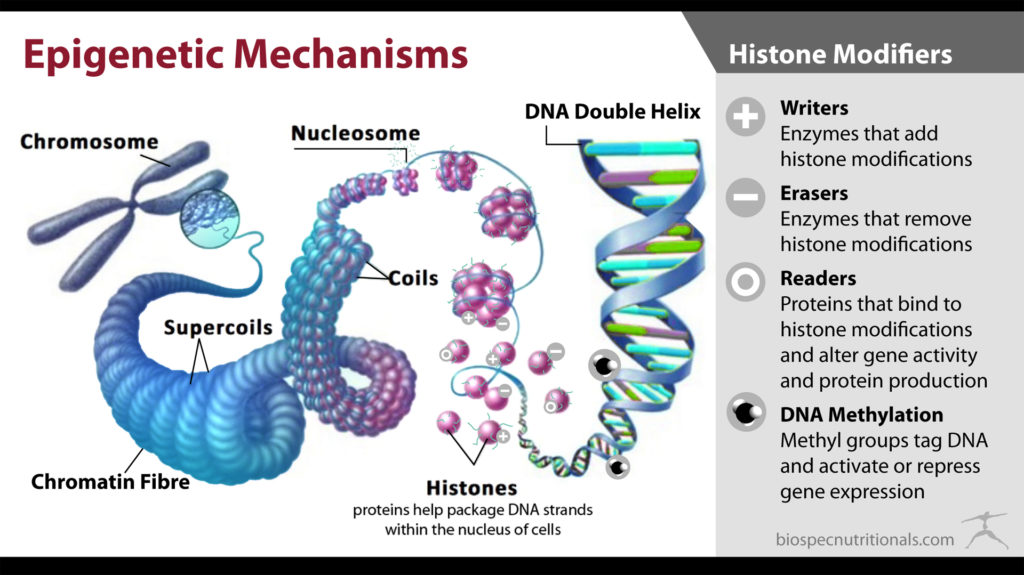
- The writers (DNMTs) are responsible for activating the addition of a methyl group to the cytosine residues of DNA strands.
- The readers (MDB, UHRF, zinc-finger proteins) function to recognize methyl groups to bind to and ultimately influence genetic expression.
- The erasers can act passively by leaving an unmethylated portion when the DNA strand splits for replication, or they can actively remove a methyl group in a series of enzymatic processes. The actions of erasers still remain unclear, but there are numerous hypotheses regarding the process.²
METHYLATION MATERIALS ¹¹ ¹² ¹³ ¹⁴
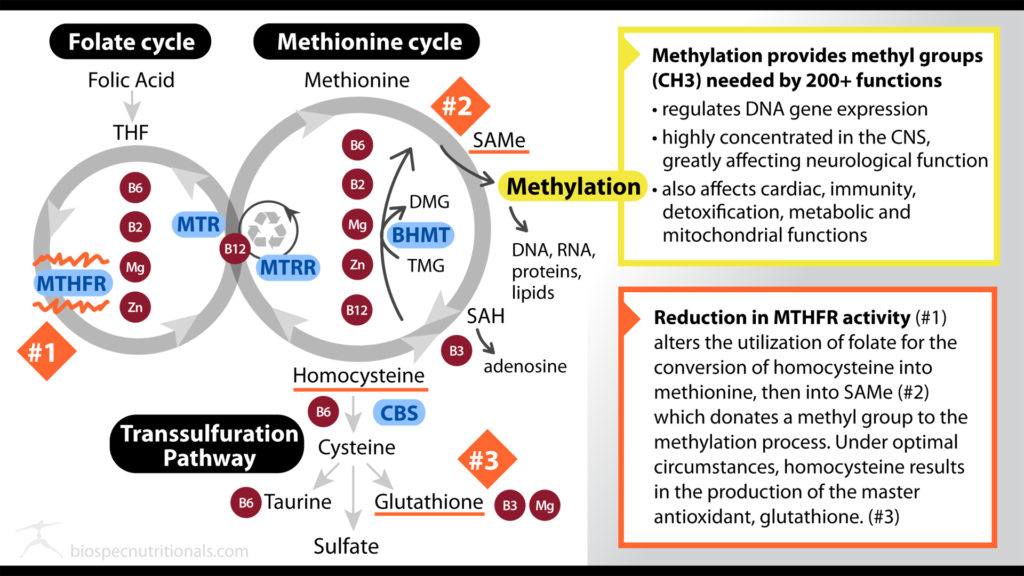
Both the Folate Cycle and the Methionine Cycle feed into one another which results in the process of methylation. In the folate cycle, folate is eventually converted to 5-methyltetrahydrofolate (5-MTHF) with the help of the enzyme methylenetetrahydrofolate reductase (MTHFR) where 5-MTHF is then able to donate a methyl group to homocysteine in the Methionine Cycle.
The major player in methylation that has been most studied is folate. Methylation is dependent upon proper metabolism of folate/folic acid in the folate cycle. It is important to note that folic acid and folate are not equivalent. Folic acid is a synthetic form of folate.
Those with methylation issues may have a difficult time converting folic acid into usable folate, leading to subsequent health issues. For this reason, it is recommended to supplement with methylated folate rather than folic acid.
Water soluble vitamins B2, B6 and B12 are also important in the Folate and Methionine Cycle:
- Vitamin B6 is a cofactor in the early stages of the Folate Cycle that turns tetrahydrofolate (THF) into 5,10-methylenetetrahydrofolate.
- Vitamin B2 then converts 5,10-MTHF into 5-methyl THF to be turned into methionine in the Methionine Cycle which is then converted to S-adenosylmethionine (SAMe) for methylation.
- Vitamin B12 is utilized in the conversion of homocysteine to methionine.
- Magnesium and Zinc are also involved in the metabolism of folate, and Methionine Cycle.
Within the Methionine Cycle, choline, Dimethylglycine (DMG) and Trimethylglycine (TMG aka betaine) are utilized to turn homocysteine into methionine. The oxidation of dietary choline creates the conversion to TMG allowing for the donation of a methyl group to homocysteine to continue the Methionine cycle.
SAMe of the Methionine cycle is the methyl group donor for methylation. Note that supplementation with SAMe can be quite costly as an approach to increase methylation in comparison to DMG/TMG supplementation.
HOW METHYLATION GOES AWRY ¹²
There are numerous factors that contribute to methylation capability. Deficiencies or excess in any of the aforementioned compounds can result in altered methylation. Other contributors to poor methylation:
- medications
- yeast
- elevated estrogens
- high histamine diet
- stress
- Lipopolysaccharides from bacterial infections (LPS)¹⁵
- heavy metals¹⁶ ¹⁷
- inflammation¹⁸
- alcohol¹⁹
It must be noted that there is the possibility of hypermethylation or hypomethylation, where the methylation pathway is sped up or slowed down, respectively. Hypermethylation can be seen in a nutshell as over-silencing of genes, whereas hypomethylation can be viewed as increased gene activity.
Hypomethylation is generally driven by the aforementioned stressors given that they require the action of the body’s metabolic and detoxification systems, thus depleting the substrates and cofactors necessary for methylation.
Hypermethylation can be caused by diets high in the amino acid methionine, elevating SAMe which drives methylation. Foods high in methionine are protein rich foods such as animal sources like meats, fish and eggs. Those with diets high in animal products, as well as protein containing nuts, seeds and grains are at higher risk of overmethylation.
⚠ NOTE: Some people react poorly to methyl donors in states of both hypo and hypermethylation. Niacin is a methyl burning compound and should be made available for those that are sensitive to methyl donors, especially via supplementation.
Alterations in methylation can lead to the elevation of homocysteine levels, called hyperhomocysteinemia which has been linked to a number of conditions related, but not limited to cardiovascular disease, neurological disorders and even cancer. ²⁰ ²¹
Knowledgeable practitioners often use homocysteine testing to evaluate the progression of the aforementioned conditions but also to gauge inflammatory load in patients as well as use it as a baseline marker to assess treatment efficacy. Ideally, homocysteine levels should be <7umol/L.
Why all the Hype on MTHFR?
An emerging topic that has gained attention in methylation research has been the effects of methylenetetrahydrofolate reductase (MTHFR) single nucleotide polymorphisms (SNPs) on methylation. Yes, that is a mouthful!
MTHFR is a gene that plays a complex role in human health, most notably in the methylation process. The MTHFR gene regulates the production of the regulatory enzyme of the same name that takes part in the metabolism of folate. The MTHFR gene has numerous variants with two specifically that have taken center stage in testing and health outcomes.
The C667T and A1298C gene variants can have genetic mutations (SNPs) that can alter the body’s ability to methylate properly and/or alter the ability to convert dietary folate/folic acid into the usable form, methylfolate.
Every gene (C667T/A1298C) consists of a pair of nucleic acids (A,T,C,G), one of each inherited by each parent. There can be a genetic mutation in one of either pair (+/-), in both of either pair (+/+), or in one of both pairs (+/-, +/-).
Genetic Variations33:
Heterozygous C667T OR A1298C: +/- One genetic mutation exists in one pair
Homozygous C667T OR A1298C: +/+ Two genetic mutations of one pair
Compound Heterozygous C667T & A1298C: +/-, +/- Both pairs with 1 mutation
Methylation Compromise by Variation Type:
C667T heterozygous (+/-): Up to 40% methylation compromise
C667T homozygous (+/+): Up to 75% methylation compromise
A1298C heterozygous (+/-): Up to 20% methylation compromise
A1298C homozygous (+/+): Up to 50% methylation compromise
Compound heterozygous (+/-, +/-): Up to 60-70% methylation compromise
MTHFR AND THE HISTAMINE CONNECTION
MTHFR is required for the reduction of intracellular histamine which requires Vitamin B2 as a cofactor. Because of this, MTHFR and histamine are inversely related. This can show up clinically as someone showing signs of histamine intolerance (elevated histamine levels), which may indicate that they are undermethylating and potentially in need of B2. Methylation support may be very beneficial to those expressing elevated levels of histamine, i.e. allergies, food intolerance, rashes, flushing, itching, profuse sweating, bloody nose, motion sickness, and much, much more.
MTHFR ISN’T THE ONLY PLAYER
The Methionine Synthase Reductase (MTRR) gene is responsible for the production of the enzyme methionine synthase reductase (MTR), which together with the enzyme methionine synthase and B12, allow for the conversion of homocysteine to methionine. 2² MTRR is especially important for the repair of B12 as a result of oxidative stress as well the reduction of elevated homocysteine levels. Those with a genetic mutation may suffer from low levels of B12 during times of increased oxidative stress.
The Betaine Homocysteine S-Methyltransferase (BHMT) gene catalyzes the conversion of trimethylglycine (betaine) to dimethylglycine and the shortcut route of homocysteine to methionine. 23 An alteration in the BHMT gene can also create an environment of elevated levels of homocysteine.
THE GOOD NEWS
Those with gene mutations (SNPs) may feel bound to a specific health outcome based on their genetic makeup. The good news is that epigenetics has shown that we can in fact alter the expression of our genes and work around any genetic pathway dysfunction with proper knowledge and understanding of the individual’s shortcomings, as well as the Folate and Methionine Cycles in relation to methylation.
Some practitioners have merely provided methyl donors for those with poor methylation or SNPs, but research has shown that over supplementation can also have adverse effects. A study found that both folate deficiency and excess were linked to DNA damage.²⁴ Because of this, testing along with a knowledgeable practitioner are key to overcoming methylation issues.
METHYLATION TESTING
Testing for genetic mutations (SNPs) can be very informative, but it’s best to also evaluate the players in methylation and their metabolites. To assess methylation capability, a practitioner should not only assess genetic factors, but also a basic serum panel which includes folate, B12, MMA (methylmalonic acid) and homocysteine to paint a bigger picture.
MMA is a better marker to evaluate B12 status due to the fact that serum B12 can fluctuate and/or may not reflect utilization within the cell. Vitamin B12 is required to metabolize MMA to succinyl-CoA for use in the energy producing Kreb’s cycle. Inadequate levels of B12 would cause an increase in MMA levels due to lack of conversion, which would be reflected by elevated levels of MMA in either serum, RBC or urine testing. The downfall to testing MMA is that it can be expensive. Some practitioners who are being cognizant of patient affordability may test for low levels of serum B12 as well as look for clinical manifestations of B12 deficiency and assume B12 deficiency if both are present.
Elevated homocysteine can be indicative of either B12 or folate deficiency, or both since they are necessary for the direct and indirect conversion of homocysteine to methionine.
A formiminoglutamic acid (FIGLU) urine excretion test, that is both sensitive and has a low rate of false positives, is also another way in which to determine folate deficiency, specifically. Metabolism of FIGLU requires folate and thus elevated levels would indicate even a minor folate deficiency.
NUTRITIONAL INTERVENTION
Ways to Improve Methylation
REDUCE NUTRITIONAL DEFICIENCIES
Focus on folate, B2, B6, B12, magnesium, and zinc. Consider a diet that incorporates foods rich in these vitamins and minerals, along with specific dietary supplements, if necessary, to support optimal methylation. Given that altered methylation affects the ability to detoxify, make sure to consume grass-fed and organic foods when possible as to reduce toxic burden.
- Sources of B9 (Folate): Dark leafy greens, beef liver, black-eyed peas, asparagus, brussel sprouts, broccoli, avocado, peas, kidney beans, orange, papaya, banana25
- Sources of B2 (Riboflavin): oats, quinoa, rice, beef, beef liver, clams, mushrooms, almonds, egg, chicken, salmon, cod, spinach, apple, kidney beans, tomato26
- Sources of B6 (Pyridoxine): chickpeas, beef liver, beef, tuna, salmon, chicken, potato, turkey, banana, bulgur, squash, nuts, rice, raisins, onion, spinach, tofu, watermelon27
- Sources B12 (Methylcobalamin): Clams, beef liver, trout, salmon, tuna, nutritional yeast, haddock, beef, dairy, ham, eggs, chicken28
- Sources of Magnesium: Bananas, Avocados, Nuts (almonds, pistachios, hazelnuts, peanuts, walnuts, cashews), seeds (chia, pumpkin), leafy greens (spinach, kelp), oats, and legumes (peas, lentils)29
- Sources of Zinc: Avocados, oysters, lobster, spinach, beans, almonds, and chia seeds30
⚠ NOTE: Vegetarianism, veganism and diets devoid of grains can make one susceptible to low levels of B2, B6 and/or B12 and thus may necessitate supplementation to support proper methylation.
SUPPORT DIGESTIVE HEALTH
First and foremost, addressing nutritional deficiencies requires optimal intestinal absorption of nutrients from dietary sources. Reducing gut lining damage with the reduction of inflammation as well as supporting the enzymatic breakdown of food is a good first step to increasing the absorption capability. Gut bacteria also play a direct role in the methylation cycle. Bifidobacterium are folate producers31 whereas the lactobacillus family are folate consumers.32 An overgrowth in either of these species (dysbiosis) can create an environment of hyper or hypomethylation, respectively.
REDUCE METHYLATION BLOCKING/COMPETITION
The previously mentioned contributors to poor methylation are what burns through methyl donors and/or prevents the methylation cycle from continuing. Decreasing exposure to toxins, infection, stress, poor diet and alcohol can significantly free up methyl donors and/or allow for optimal methylation for normal, everyday bodily functions.
CONSERVE METHYL DONORS AND REBALANCE METHYLATION
Compounds that help to conserve methyl donors in those struggling with methylation imbalance are creatine, phosphatidylcholine, carnitine and melatonin. Methylation adaptogens, coined by Dr. Michael Stone, are also an option to balance both hyper and hypomethylation. These include curcumin (from turmeric), sulforaphane (from cruciferous veggies), quercetin (from red onion and kale), rosmarinic acid (from rosemary), betanin (from beets), anthocyanins (from dark berries) and lycopene (from tomatoes).
METHYLATION & EPIGENETICS
Summary
Methylation is an epigenetic process that attaches a methyl group to a DNA strand, affecting the expression of genetic information without altering the actual DNA structure.
Methylation plays a part in so many physiologic processes and when altered, can contribute to the progression of disease, including but not limited to, cardiovascular disease, neurological disease and even cancer.
Factors such as environmental toxins, heavy metals, medications, stress, diet, infection and inflammation can all affect methylation capacity. Because methylation issues can affect detoxification status, it is imperative to reduce exposure to toxins along with making necessary lifestyle changes which can dramatically improve methylation status and overall health.
Increased necessity for detoxification and increased states of stress may cause someone to present as a hypomethylator, whereas someone who partakes in a high protein diet (namely animal proteins) may cause hypermethylation.
Histamine intolerance (allergies/food sensitivities) can be an indication of hypomethylation (MTHFR SNP) and/or a B2 deficiency.
SNPs of the MTHFR gene are the most notable of genes that alter methylation, but MTRR and BHMT SNPs can also result in methylation dysfunction.
Evaluation of genetic factors, along with the assessment of methylation cofactors and byproducts are a logical approach to personalized treatment for someone with methylation issues. These tests include serum B2, B6, folate, B12, Methylmalonic Acid (MMA), Homocysteine and FIGLU. Make sure to retest 60-90 days post therapeutic intervention to assess for any necessary changes in protocol.
SUPPORT OPTIMAL METHYLATION
Action Steps
Dietary changes should include increased consumption of B2, B6, folate, B12, magnesium and zinc along with supplementation (if necessary) where deficiencies are present. Include dietary methylation adaptogens (curcumin, betanin, rosmarinic acid, anthocyanins, sulforaphane, quercetin and lycopene) to aid in balancing both hyper and hypo methylation.
Avoid folic acid in supplements and fortified foods. When supplementing, use methylated forms of B9 and B12 vitamins: methylfolate and methylcobalamin.
Niacin can help to burn methyl donors and reduce adverse effects, while figuring out safe dosing levels for those who respond poorly to high-dose methyl supplementation.
⚠ Note that high-dose methyl donors should not be a long term approach to methylation dysfunction.
Creatine, melatonin, phosphatidylcholine and carnitine can help to conserve methylation by providing extra methyl donors.
Specific dietary plans may include an anti inflammatory diet for both hypo and hyper methylators. A diet low in histamine producing foods may be beneficial for someone who under methylates, whereas a diet low in methionine may be beneficial for someone who has issues with hypermethylation.
Reduce toxin exposure and overall toxic load. Consume grass-fed and organic whenever possible.
Find ways in which to either reduce stress (mental, physical and emotional), or find stress management strategies to create a more balanced stress response to unavoidable stress.
Address any underlying infection, whether acute or chronic.
Address gut health through dietary changes, removing reactive foods, enzymatic support and probiotic supplementation of proper microbial species.
References
- Felsenfeld G. A Brief History of Epigenetics. Cold Spring Harb Perspect Biol. 2014 Jan; 6(1): a018200.
- Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38(1):23-38. doi:10.1038/npp.2012.112
- Kim GH, Ryan JJ, Archer SL. The role of redox signalling in epigenetics and cardiovascular disease. Antioxidants & Redox Signaling, 2013;18(15):1920-1936.
- Martinez SR, Gay MS, Zhang L. Epigenetic mechanisms in heart development and disease. Drug Discov Today 2015;20(7):799-811.
- Wu H, Deng Y, Feng Y, Long D, Ma K, Wang X, Zhao M, Lu L, Lu Q. Epigenetic regulation in B-cell maturation and its dysregulation in autoimmunity. Cellular & Molecular Immunology. 29 January 2018; 15: 676–684(2018).
- Weisberg I,Tran P, Christensen B, Sibani S, Rozen A Second Genetic Polymorphism in Methylenetetrahydrofolate Reductase (MTHFR) Associated with Decreased Enzyme Activity. Molecular Genetics and Metabolism. July 1998;64(3):169-172.
- Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev. 2008;13(3):216-226.
- Keating S, El-Osta Epigenetics and Metabolism. Circulation Research. 2015;116:715–736.
- Hoelzer K, Shackelton LA, Parrish CR. Presence and role of cytosine methylation in DNA viruses of animals. Nucleic Acids Res. 2008;36(9):2825-2837. doi:10.1093/nar/gkn121
- Jeudy, S., Rigou, S., Alempic, J. et al. The DNA methylation landscape of giant viruses. Nat Commun 11, 2657 (2020). https://doi.org/10.1038/s41467-020-16414-2
- Anderson OS, Sant KE, Dolinoy Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation.The Journal of Nutritional Biochemistry. August 2012; 23(8): 853-859.
- Lynch B. Methyl Group, Methylation, Methyl Trapping: What Are They? Article retrieved 26 July 2020. https://www.drbenlynch.com/methyl-group-methylation-methyl-trapping-what/.
- Watani M, Ikegami K, Kremenska Y, et al. Dimethyl sulfoxide has an impact on epigenetic profile in mouse embryoid body. Stem Cells. 2006;24(11):2549-2556.
- Thaler R, Spitzer S, Karlic H, Klaushofer K, Varga F. DMSO is a strong inducer of DNA hydroxymethylation in pre-osteoblastic MC3T3-E1 cells. Epigenetics. 2012;7(6):635-651. doi:10.4161/epi.20163
- Novakovic B, Habibi E, Wang SY, Martens JHA, Logie C, Stunnenberg HG. 𝛃-Glucan Reverses the Epigenetic State of LPS-Induced Immunological Tolerance. J Cell. Article. 17 Nov 2016; 167(5):1354-1368. DOI: https://doi.org/10.1016/j.cell.2016/09/034.
- Ryu HW, Lee DH, Won HR, Kim KH, Seong YJ, Kwon SH. Influence of toxicologically relevant metals on human epigenetic regulation. Toxicol Res. 2015;31(1):1-9. doi:10.5487/TR.2015.31.1.001.
- Willhite CC, Karyakina NA, Yokel RA, et al. Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Crit Rev Toxicol. 2014;44 Suppl 4(Suppl 4):1-80. doi:10.3109/10408444.2014.934439
- Smith JA, Zhao W, Wang X, et al. Neighborhood characteristics influence DNA methylation of genes involved in stress response and inflammation: The Multi-Ethnic Study of Atherosclerosis. Epigenetics. 2017;12(8):662-673. doi:10.1080/15592294.2017.1341026.
- Cervera-Juanes R, Wilhelm LJ, Park B, Grant KA, Ferguson B. Genome-wide analysis of the nucleus accumbens identifies DNA methylation signals differentiating low/binge from heavy alcohol drinking. Alcohol. 2017;60:103-113. doi:10.1016/j.alcohol.2016.11.003.
- Ansari R, Mahta A, Mallack E, Luo JJ. Hyperhomocysteinemia and neurologic disorders: a review [published correction appears in J Clin Neurol. 2015 Jan;11(1):106]. J Clin Neurol. 2014;10(4):281-288. doi:10.3988/jcn.2014.10.4.281
- Yates A. Epigenetics, Methylation and Cancer. National Foundation for Cancer Research. 24 Jul 2018. https://nfcr.org/blog/epigenetics-methylation-and-cancer/
- MTRR Gene. Genetics Home Reference. National Institute of Health. 4 Aug 2020. https://ghr.nlm.nih.gov/gene/MTRR#normalfunction
- BHMT betaine–homocysteine S-methyltransferase [homo sapiens (human)]. National Institute of Health. 1 Aug 2020. https://www.ncbi.nlm.nih.gov/gene/635
- Ortbauer M, Ripper D, Fuhrmann T, Lassi M, Auernigg‐Haselmaier S, Stiegler C, König J. Folate deficiency and over‐supplementation causes impaired folate metabolism: Regulation and adaptation mechanisms in Caenorhabditis elegans . Mol. Nutr. Food Res. 2016;60: 949-956.
- Fact Sheet for Health Professionals. National Institute of Health. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/
- Fact Sheet for Health Professionals. National Institute of Health. https://ods.od.nih.gov/factsheets/Riboflavin-HealthProfessional/
- Fact Sheet for Health Professionals. National Institute of Health. https://ods.od.nih.gov/factsheets/VitaminB6-HealthProfessional/
- Fact Sheet for Health Professionals. National Institute of Health. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/
- Fact Sheet for Health Professionals. National Institute of Health. https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/
- Fact Sheet for Health Professionals. National Institute of Health. https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/
- Folate Production by Bifidobacteria as a Potential Probiotic Property. Pompei A, Cordisco L, Amaretti A, Zanoni S, Matteuzzi D, Rossi M. Applied and Environmental Microbiology. Dec 2006;73 (1) 179-185; DOI: 10.1128/AEM.01763-06
- Ross M, Amaretti A, Raimondi S. Folate Production by Probiotic Bacteria. Nutrients. 01 Dec 2011; 3(1):118-134. DOI:10.3390/nu3010118
- Liew SC, Gupta ED. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: Epidemiology, metabolism and the associated diseases. European Journal of Medical Genetics. January 2015; 58(1):1-10.
Related Resources & Links
» What is MTHFR? | Dr Berg Explains in simple terms (5min video)
BioSpec Nutritionals is not affiliated nor endorsed by any individuals, organizations, or other entities related to off-site links published herewithin.
Related Products & Offers
— NUTRACEUTICAL SUPPORT —
Methylation, Detox & Elimination
Methyl-Ease HP
An advanced, high potency formula designed to support proper methylation and ease symptoms associated with severe methylation compromise, and Peripheral Neuropathy.*
Methyl B-12 2000
A highly absorbable form of Methylcobalamin (vitamin B-12) plus Methyl Folate (activated folic acid) in a great tasting chewable tablet.
Mag Glycinate 510
A clinically significant dose of Magnesium, which is central in cellular energy production, muscle contraction, nerve impulses and maintenance of proper bone mineralization.* Also includes Glycine, an amino acid shown to support healthy kidney and liver function, and nervous system health.*
Bio-Zinc 50
A high-potency zinc complex for immune, antioxidant, and anti-inflammatory support.* Among its many roles, Zinc is vital for DNA synthesis, thyroid and bone metabolism, skeletal development, visual health, hearing, taste, and helps modulate oxidative and inflammatory processes, and various aspects of innate and adaptive immunity.
Bio-E-400
A highly absorbable softgel with 100% natural source Vitamin E.
Vitamin E is the body’s primary fat soluble antioxidant with the ability to reduce oxidative stress within the lipid membranes.*
Co-Q-Sol CF
Enhanced absorption Coenzyme Q-10, crystal-free ubiquinone with Vitamin E.*
Mito-Detox III (60 or 120 caps)
Helps optimize cellular energy production and
supports balanced detoxification pathways.*
Glyco Stress II
A unique formula providing Vitamins, Minerals and specific Herbs to support the metabolism of all major macronutrients, and promote healthy glucose levels.
— ONGOING OFFERS —
Free Shipping
Get FREE shipping when you order direct from BioSpec Nutritionals. Applies to all retail and wholesale orders. No code needed.
— PRACTITIONER OFFERS —

Free Literature & Other B2B Benefits
- FREE educations brochures and cards, shipping free of charge
- FREE product samples, also applies to CBD
- FREE price lists, for patients and office use
- Wholesale Pricing for Practitioners
B2B benefits apply to registered practitioners only.
Limits restrictions apply. Contact BioSpec for details.
Medical Disclaimer: This content is for informational and educational purposes only. It is not intended to provide medical advice or take the place of such advice or treatment from a personal physician. All readers/viewers of this content are advised to consult their doctor or qualified health professional regarding specific health questions. Neither BioSpec Nutritionals, Practitioner Supply nor the publisher of this content takes responsibility for possible health consequences of anyone reading or following the information in this educational content. All viewers of this content, especially those taking prescription or over-the-counter medications, should consult their physicians before beginning any nutrition, supplement or lifestyle program.