Multiple Sclerosis (MS) Education & Awareness Month
By Dr. Melissa Mitchell, DC, CFMP
News & Science Behind Formulas | Gut & Immune Products | Resources & Links
According to a 2017 National MS Society study, there are nearly 1 million people living with MS in the United States. The incidence and prevalence of MS may potentially be higher based on discrepancies in tracking data and lapses in communication between government health agencies and medical communities.²
Women are three times more likely to develop MS than men, and more recent research found that the occurrence in African American women is much higher than previously reported.³
Table of contents
What Causes MS
The specific causes of MS have yet to be fully understood, though it is believed that both genetic and environmental factors influence disease incidence and progression. Because of the disease prevalence in people of northern European ancestry and in countries furthest from the equator, it has raised suspicion that vitamin D deficiency may play a role in the development and/or progression of MS.
The National MS Society is currently funding research and partnering with institutions like Johns Hopkins and Cleveland Clinic to further elucidate the role that vitamin D plays in MS.⁴⁻⁵ A study in 2011 found that participants who had serum 25-hydroxyvitamin D levels of ≥ 75 nmol/L had a 61% decreased risk of MS.⁵ Another interesting study found a correlation between mothers exposed to more sunlight while pregnant and their offspring who were at lower risk of developing MS.⁶
While vitamin D status remains an important consideration in MS pathology, current research has focused largely on the relationship involving digestive health and autoimmunity, with a number of top scientists and medical researchers directing their efforts in this area. World-renown physician and medical researcher Alessio Fasano, for example, has promoted the observation that three essential factors underlie the initiation of autoimmunity, and include:
- a triggering event (i.e. infection/pathogen exposure)
- genetic susceptibility to a given condition
- significant permeability of the small intestine
Among the major factors identified, intestinal permeability tends to be overlooked in the mainstream medical community, partially due to a lack of knowledge and expertise on the subject. However, the influence of intestinal permeability in autoimmunity and other seemingly unrelated conditions (i.e. cardiovascular disease) continues to gain greater acceptance with accumulating research. It’s worth noting that the influence of gastrointestinal dysfunction in immune dysregulation and disease becomes much easier to digest (no pun intended) when considering the role of Gastrointestinal Associated Lymphoid Tissue (GALT).
GALT resides throughout the intestine and houses roughly
70% of the body’s entire immune system.
GALT is comprised of several types of lymphoid tissue that houses immune cells to counter the continuous exposure of pathogens passing through the digestive tract.⁷ To better elucidate the bidirectional and interactive influences of the immune and gastrointestinal systems on autoimmunity, especially MS, it is prudent to first become acquainted with the digestive (intestinal) barrier that selectively absorbs essential nutrients while protecting the internal environment of the body from pathogens and other potentially harmful opportunistic substances.
Intestinal Barrier Function
The intestinal barrier (a.k.a. mucosal barrier) is a term established more recently by gastroenterologists, immunologists, and microbiologists to emphasize the protective component of the GI tract that shields against harmful bacteria or invasion of other microorganisms and their toxins. The intestinal barrier consists of multiple layers including an external physical barrier of epithelial cells and mucus, and an inner functional region (lamina propria) of immune cells and humoral elements that form an immunological barrier. While assimilation of nutrients by intestinal cells remains an essential function of the GI tract, it’s role as a physical barrier remains essential to immune regulation and prevention of autoimmunity.
When examining the physical component of the intestinal barrier, it includes a single layer of epithelial cells (known as enterocytes) with finger-like projections (villi) that increase intestinal surface area to allow for maximal absorption of nutrients. This layer of intestinal cells is connected by ‘tight junctions’ that participate in assimilation by selectively allowing the passage of nutrients, electrolytes/chemical elements and water, while preventing the absorption of toxins, antigens, microbes, and their harmful byproducts. Various immune cells reside within and around different regions of the intestinal barrier, which functions as a lymphoid organ of the gastrointestinal immune system, in addition to its well-known roles in digestion and absorption. The gastrointestinal immune system activates and instructs immune cells to eliminate or destroy harmful substances found within the digestive tract.⁸
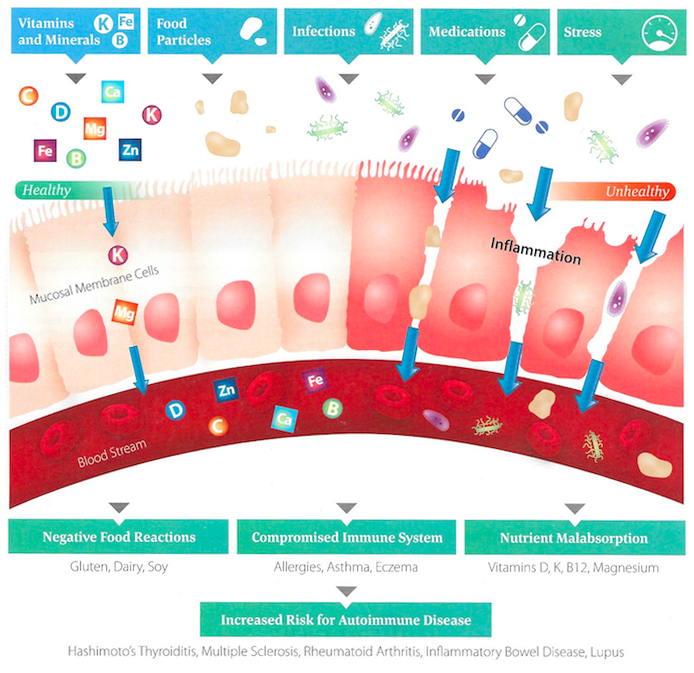
Intestinal Permeability, Inflammation & Autoimmunity
Factors such as poor dietary choices (i.e. processed foods, refined carbohydrates, gluten, etc.), medications, alcohol, stress, environmental toxins, infectious load and hormonal/neurologic/metabolic imbalances all can take a toll on the intestinal barrier, leading to a reduction of the absorptive surface area of enterocytes, a reduction of digestive enzyme production, and the breakdown of intestinal lining integrity. This can severely reduce the body’s ability to utilize essential nutrients that supply energy for tissue maintenance and repair. Chronic exposure to these harmful dietary, lifestyle, and environmental factors can induce intestinal permeability, or “leaky gut”, which is a breach in tight junctions between cells (paracellular) or through cells (transcellular), allowing for undigested food particles, toxins, and microbes and their byproducts to enter the bloodstream and cause excessive immune and inflammatory responses.⁹ Remember, only properly digested food molecules and other familiar substances are allowed to enter the bloodstream without consequence.
The ongoing release of systemic inflammatory substances (chronic inflammation) in response to a leaky gut can cause symptoms such as joint pain, fatigue, depression, brain fog, muscle aches and much more. Previously injured, degraded, or susceptible tissue becomes vulnerable to the effects of chronic inflammation, leading to the release of more inflammatory substances and feeding a vicious cycle.¹º
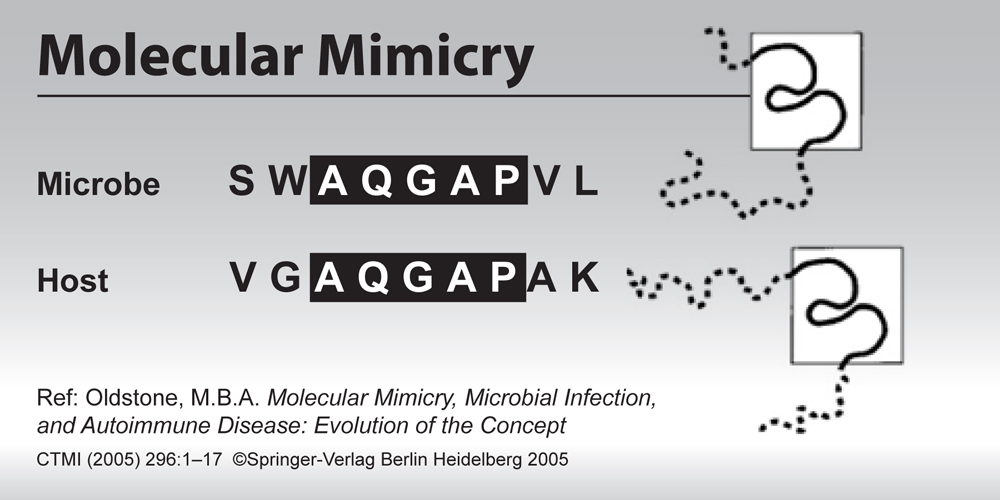
With regard to autoimmunity, intestinal permeability can significantly increase the likelihood of a process known as molecular mimicry. In simple terms, molecular mimicry occurs when microbes and/or undigested food particles (amino acid chains) pass through the damaged intestinal barrier unabated and are mistaken for self-tissue based on their molecular similarities. Essentially, immune cells that are programmed to attack unrecognized invaders begin to associate structurally similar self-tissue (i.e. myelin sheath) with undigested proteins and other substances that would typically never access the bloodstream. This, along with chronic inflammation, creates an autoimmune response in which the immune system can no longer distinguish between self and non-self, thus creating antibodies that facilitate the attack of T-cells against self-tissue. As such, any treatment protocol aimed at addressing the damage caused by systemic inflammatory and autoimmune processes must also consider therapies focused on healing the intestinal barrier.
MS, Infection & Gut Bacteria
Aside from undigested proteins and other substances that reach circulation as a result of leaky gut, other infectious pathogens have shown to present with similar molecular sequencing of tissues susceptible in the progression of MS. Infectious agents identified include chlamydia, Epstein-Barr virus, coronavirus, hepatitis B, herpesvirus, pseudomonas, rubella and haemophilus influenzae, all of which can create molecular mimicry against myelin components.¹¹ However, while infectious agents can trigger autoimmunity by various means, it’s essential to acknowledge the protective roles of beneficial intestinal bacteria, which serve to preserve the integrity of the intestinal barrier, in addition to preventing the invasion of harmful microorganisms that stimulate and exacerbate an immune response.
In a systematic review of experimental animal studies, it was shown that intestinal implantation of specific bacteria species could either turn on inflammatory proteins and/or stimulate particular portions of the immune system that incite experimental autoimmune encephalomyelitis (EAE), the most commonly used experimental model to study MS. The review found that it wasn’t simply the presence of harmful bacteria that caused an autoimmune response, but also that lack of beneficial bacteria could increase MS susceptibility. By colonizing the intestines with specific microbial species, researchers were able to upregulate anti-inflammatory proteins and reduce the activation of the immune response that leads to EAE.¹²
Immune Imbalance
Another area of interest in the development and treatment of autoimmune disease involves the balance of T-helper (Th) cell activity. Within the adaptive immune response exists a subset of white blood cells that play an important role in autoimmunity called T-helper (Th) cells. These cells mature in the thymus and later develop a specific immune response to different antigens (i.e. bacteria, viruses, parasites, allergens, mold, reactive foods) based on their interactions with other immune cells identified as antigen presenting cells (APCs).
Activated T-helper cells can be classified by the type of response they elicit. Some T-helper cells will respond primarily to viral and bacterial infection (Th1 cells), whereas others are responsive to worms, parasites, and allergens (Th2 cells). It is important to recognize that each of these responses will involve different signaling molecules, called cytokines, that help to direct the activities of other immune cells for the effective elimination of a threat. In terms of using this information clinically, it’s critical to understand how different responses can share an inverse relationship. For example, an elevated Th1 response to a viral infection will subsequently result in a diminished Th2 response and vice versa. This has to do with how the cytokines produced within a specific response, like Th1 for example, can disable the production of cytokines produced by Th2 cells, with only one response expressing dominance at any given time.
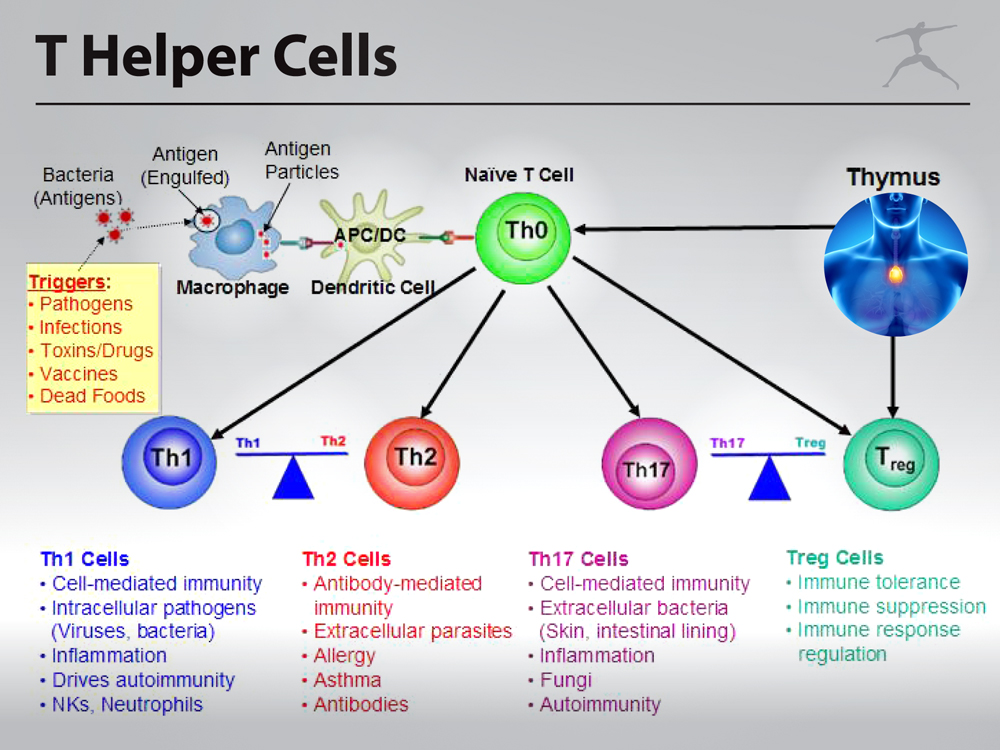
The same can be said of other T-helper cell responses like Th17 and Treg (as in T-regulatory). Th17 and Treg cells share a common signaling pathway as Th17 cells produce cytokines that are proinflammatory and contribute significantly to autoimmunity, whereas Treg cells produce anti-inflammatory cytokines that suppress the activity of a variety of immune cells, and therefore inhibit immune responses. Considering that Th1 and Th17 help to drive the autoimmune response, clinical interventions involving nutrition should focus on potentially increasing Th2 cell activity to reduce Th1 cytokines, however, increasing Treg cell activity is likely to produce the greatest benefit as Treg cells are also capable of balancing Th1/Th2 responses and suppressing Th17, the most significant contributor to autoimmunity.¹³
Vitamin D and the Upregulation of T Regulatory Cells
Fortunately, a simple way in which Treg cells can be increased is through Vitamin D supplementation. With the guidance of a knowledgeable practitioner, an individual can evaluate their 25-OH Vitamin D3 levels through a simple blood test to see how much Vitamin D3 they should be taking to reach optimal levels. Optimal levels of 25-OH Vitamin D3 are minimum 80 ng/dL for someone who is suffering with MS and/or has chronic inflammation. Supplementation of Vitamin A in combination with Vitamin D may enhance vitamin D receptor activity to help modulate immune balance.¹⁴
Benefits of Essential Fatty Acids
Omega-3 fatty acids have been found to preserve the myelin sheath and nerve impulse conductivity as well as reduce inflammation, a common contributing factor in MS. A 2013 study found that omega-3 supplementation protected against destruction of the hippocampal region of the brain.¹⁵ A recent literature review showed that supplementation with 4g daily helped with remitting relapse and progression of the disease as well as increased the quality of life in patients suffering from MS.¹⁶
Probiotics and Diet for Intestinal Permeability
In addition to addressing dietary, environmental, and lifestyle factors that may be contributing to intestinal permeability, and thus MS disease activity, current patients or those at risk (based on genetic and other predisposing factors) should consider supporting intestinal integrity and digestive health with a combination of probiotics and Gastri-Ease HP from BioSpec. Gastri-Ease includes a combination of clinically studied ingredients supported for their effects on several aspects of intestinal barrier structure and function.
Additional information is available in the following links (see ‘Resources & Links’ section below) that support the use of specific dietary and toxin elimination strategies for improving intestinal permeability and autoimmunity.
References:
- National Multiple Sclerosis Society. What is Myelin? Viewed March 3, 2020. https://www.nationalmssociety.org/What-is-MS/Definition-of-MS/Myelin
- National Multiple Sclerosis Society. How Many People Live with MS? Viewed March 3, 2020. https://www.nationalmssociety.org/What-is-MS/How-Many-People
- National Multiple Sclerosis Society. Who Gets MS? (Epidemiology). Viewed March 3, 2020. https://www.nationalmssociety.org/What-is-MS/Who-Gets-MS
- National Multiple Sclerosis Society. Vitamin D. Viewed March 3, 2020. https://www.nationalmssociety.org/Research/Research-News-Progress/Vitamin-D
- Salzer J, Hallmans G, Nyström M, Stenlund H, Wadell G, Sundström P. Vitamin D as a protective factor in multiple sclerosis. Neurology. 2012;79:2140–2145.
- Dobson R, Giovannoni G, Ramagopalan S. The month of birth effect in multiple sclerosis: systematic review, meta-analysis and effect of latitude. J Neurol Neurosurg Psychiatry. 2013;84:427–432.
- Vighi G, Marcucci F, Sensi L, Di Cara G, Frati F. Allergy and the gastrointestinal system. Clin Exp Immunol. 2008 Sep; 153(Suppl 1): 3–6.
- Romero S Cotoner A, Camacho P, Bedmar C, Vicario M. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig. 2015 Nov;107(11):686-96.
- Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42(1):71-78.
- Garn H, et. al. Current concepts in chronic inflammatory diseases: Interactions between microbes, cellular metabolism, and inflammation. Journal of Allergy and Clinical Immunology. July 2016;138 (1), pp. 47-56.
- Jane E.LibbeyLori L.McCoyRobert S.Fujinami. Molecular Mimicry in Multiple Sclerosis. International Review of Neurobiology. 2007;Volume 79, Pages 127-147.
- Brady D. Open Journal of Rheumatology and Autoimmune Diseases. 2013;Vol.3 No.1, Article ID:27948
- Charlton B, Lafferty KJ. The Th1/Th2 balance in autoimmunity. Current Opinion in Immunology.Volume 7, Issue 6, December 1995, Pages 793-798.
- Issazadeh-Navikas S, Teimer R, Bockermann R. Influence of Dietary Components on Regulatory T Cells. Mol Med. 2012;18(1): 95-110.
- AlAmmar W, Albeesh F, Ibrahim L, Algindan Y, Yamani L & Khattab R. Effect of omega-3 fatty acids and fish oil supplementation on multiple sclerosis: a systematic review, Nutritional Neuroscience. 2019;DOI: 10.1080/1028415X.2019.1659560.
- Pu H1, Guo Y, Zhang W, Huang L, Wang G, Liou AK, Zhang J, Zhang P, Leak RK, Wang Y, Chen J, Gao Y. Omega-3 polyunsaturated fatty acid supplementation improves neurologic recovery and attenuates white matter injury after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2013 Sep;33(9):1474-84.
- Oldstone, M.B.A., Molecular Mimicry, Microbial Infection, and Autoimmune Disease: Evolution of the Concept. Faseb j, 1998 12(13): p. 1255-65.
SUMMARY
- Intestinal permeability (IP, Leaky Gut) increases the likelihood of MS development. If left untreated, multiple autoimmune disorders can develop
- IP can result from poor dietary choices, medications, alcohol, stress, environmental toxins, infectious load and hormonal/neurologic/metabolic imbalances
- Infectious agents and their byproducts can increase MS risk
- Lack of microbial strains in the digestive system can increase the risk of MS
- Molecular mimicry is a process in which the immune system identifies host tissue as a foreign invader due to the similarity in the molecular makeup of the foreign substance and the host tissue. This process is a result of intestinal permeability and chronic inflammatory load
- Immune imbalance favoring Th1 and Th17 can result in the development and progression of MS
- Upregulating T regulatory cells can help to balance the Th1/Th2/Th17 portion of the immune system
- Vitamin D is often low in those suffering with MS. Vitamin D can help to upregulate Treg cells
- Levels of 25-OH Vitamin D3 at a minimum of 80 ng/dL have been shown to be helpful for those at risk of developing MS or for those who suffer
- Vitamin D and Vitamin A taken together help to enhance Vitamin D receptor activity
- Omega-3 fatty acids protect against degradation of the myelin sheath, loss of nerve transmission conduction and destruction of the hippocampus
- Omega-3 helps to reduce inflammation
- IP-Ease and probiotics to support intestinal barrier integrity and digestive function
Fortify the Gastrointestinal and Immune System with an Evidence-Based Approach
THE 4-R GUT PROTOCOL
Address Gut Dysbiosis at its Core
_________
1
REMOVE
GI Stressors & Harmful Microorganisms
Consider Para-Clear to help detoxify and cleanse the intestinal tract. May be used against a wide variety of harmful intestinal microorganisms.*
2
REPLACE
Beneficial Bacteria Species
To support digestive and immune function in the GI tract, consider robust probiotic formulas like Probiotic-5 or Ultra-Biotic 50.
3
RESTORE
Digestive Constituents & Enzyme Activity
Lack of stomach acid and enzyme activity can contribute to problems locally in the GI tract and systemically if not addressed. If symptoms of poor digestion persist, consider Bio-Acid HP and/or Bio-Enzyme Daily.
4
REPAIR
The Instestinal Barrier
Consider Gastri-Ease HP to promote healthy intestinal structure and function, an essential consideration for a competent immune system.
+RELIEF
When constipation is an issue,
consider a formula high in magnesium such as Mag Glyincate 510.
IMMUNE & ANTIOXIDANT SUPPORT
Fortify the Immune System with an Evidence-Based Approach
_________
COBOTIC
A foundational blend of ingredients for healthy immune function
Cobotic includes many of the most well-studied vitamins and minerals known to enhance a diverse range of immune activities when taken at clinical doses.
VITAMIN A & D
Support against acute and chronic illness
Vitamin A and Vitamin D remain two of the most well-researched vitamins that work to support short- and long-term health through modulation of innate, adaptive, and inflammatory immune activities.
Consider these BioSpec formulas:
Vitamin A, D3-1000, D3-5000, and Vitamin A&D
VITAMIN C
The most important extracellular antioxidant
In addition to its role as an antioxidant, vitamin C is known to shorten the duration of cold and flu when taken in sufficient doses.*
Consider these Vitamin C formulas:
Raspberry C-5000 Chewable and Buffered C-1000
Other Beneficial Supplements
For mitigating oxidative and inflammatory processes, consider adding Bio-E 400, Co-Q-Sol CF 100, Astaxanthin, and/or any of BioSpec’s Omega-3 Fish Oil Products.
Related Resources & Links
BioSpec Nutritionals is not affiliated or endorsed by any of the organizations related to off-site links shared in this newsletter
Medical Disclaimer: This content is for informational and educational purposes only. It is not intended to provide medical advice or take the place of such advice or treatment from a personal physician. All readers/viewers of this content are advised to consult their doctor or qualified health professional regarding specific health questions. Neither BioSpec Nutritionals, Practitioner Supply nor the publisher of this content takes responsibility for possible health consequences of anyone reading or following the information in this educational content. All viewers of this content, especially those taking prescription or over-the-counter medications, should consult their physicians before beginning any nutrition, supplement or lifestyle program.