Diabetic Neuropathy: Pathophysiology and Therapeutic Approaches
By Dr. Melissa Mitchell, DC, CFMP
Peripheral Neuropathy Awareness Week is observed in early May each year. An estimated 30 million people in the United States alone experience symptoms of peripheral neuropathy, which is a condition most commonly related to diabetes. Considering diabetes mellitus (DM) is the most common etiology, it is important to understand symptoms, influential factors and therapeutic approaches to address Diabetic Peripheral Neuropathy (DPN) in particular.
NEUROPATHY SYMPTOMS¹
- Sensory changes: numbness, tingling, throbbing, stabbing, burning, sharpness, electrical sensations, pain, a feeling of wearing socks or gloves
- Muscular changes: weakness, twitching, spasms, cramping, paralysis, difficulty walking/moving, loss of grip strength, loss of muscle tone, difficulty with fine motor skills
- Organ dysfunction: Issues with urination, sexual dysfunction, digestive issues
- Cardiovascular effects: low blood pressure, abnormal heart rate, lightheadedness/dizziness/fainting upon standing
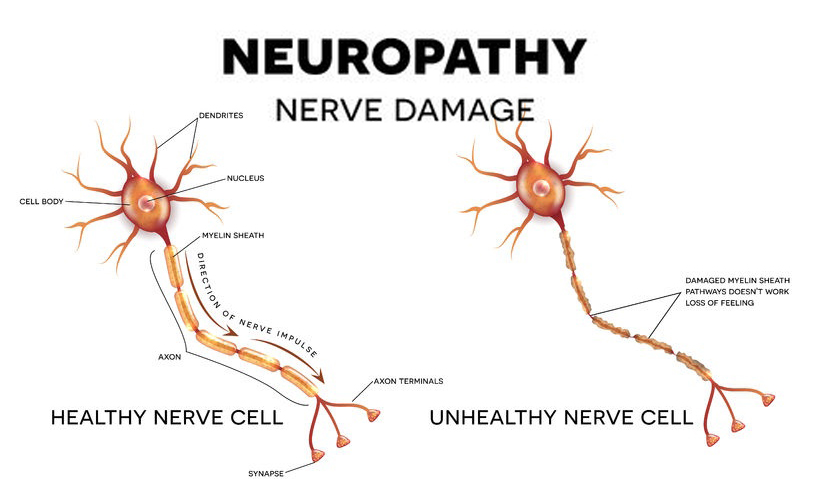
Neuropathy, often referred to as peripheral neuropathy, is a condition resulting from damaged or dysfunctional nerves. Because neuropathy can affect any portion of peripheral nerves, sensory (the five senses), motor (muscular activity) and autonomic function (activity without direct thought control) can all be affected. As it is currently understood, there are numerous causes related to the pathophysiologic development of neuropathy with the most prevalent being from blood sugar dysregulation commonly known as diabetes. In this article we will focus in on the most ubiquitous cause of peripheral neuropathy and the concomitant factors related to diabetes and diabetic neuropathy.
THE ROLE OF DIABETES IN NEUROPATHY
In order for nerve cells to function properly and maintain their integrity, they require adequate blood supply, nutrition (vitamins, minerals, essential fatty acids) and energy in the form of optimal levels of glucose. With chronically elevated blood glucose levels, numerous biochemical reactions occur that not only damage the neural tissues but also reduce the electrical conductivity of nerve transmissions.
Key Players in the Progression of Diabetic Neuropathy
It still remains unclear as to the exact pathophysiological development of diabetic neuropathy, but there are many theoretical influences that may play a role.
Some of these factors include, but are not limited to:
Reactive Oxygen Species, Oxidative Stress and Mitochondrial Dysfunction²·³
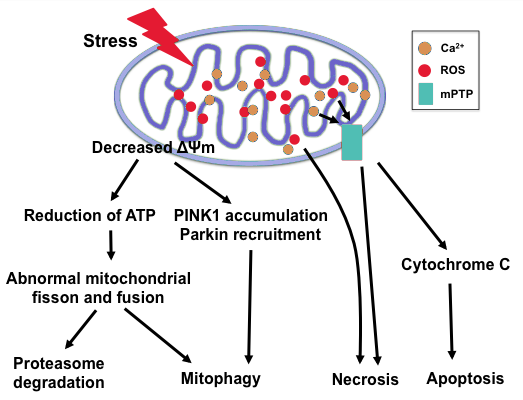
Energy production of ATP that occurs within the mitochondria of a cell creates unstable cellular byproducts known as reactive oxygen species (ROS). These ROS (oxidants) have the ability to oxidize other molecules by removing electrons in the presence of oxygen to increase their stability whilst inflicting collateral damage to nearby cellular structures, cells and tissues. Under normal circumstances when there is a balance between oxidants and antioxidants, these free radicals serve numerous beneficial functions, but an imbalance in the production of ROS creates a state of oxidative stress.
The body’s inability to negate the effects of oxidative stress is the leading theory as to the progression of diabetic neuropathy. Oxidative stress can be likened to the browning of an apple once it has been cut or bitten into, exposing the flesh to oxygen causing accelerated cellular aging. These unstable and reactive molecules in large quantities can do significant damage to cellular membranes, proteins and DNA within the mitochondria, creating mitochondrial dysfunction and eventual loss of ATP production. This can lead to cellular dysfunction, abnormal cell replication, cell mutation and/or ultimate cell death.
The body’s natural defense against oxidation are antioxidants like glutathione, Vitamin E and Vitamin C. When antioxidant availability is low, oxidative stress becomes the dominant force with proliferation of ROS leading to dysfunctional neurons and altered blood flow of which are hallmark characteristics in diabetic neuropathy.
Microvascular Changes²
It is documented in studies that the reduced perfusion of blood from the capillaries in diabetics due to changes in microvascular structure and functionality not only reduces the blood supply to neural tissue, but to the skin as well. These changes promote swelling and pressure within the nerves leading to hypoxia and nerve loss, although the theory of hypoxia is debatable in the literature and thus has been termed “pseudohypoxia.”⁴ Consequently, the degenerative and regenerative ability of axons becomes compromised in those suffering with pain.
Damaged Nerve Endings²
The functionality of nerve tissue is reliant upon electrical charges to create what is called an “action potential.” This electrical charge (in the form of an ion attached to sodium, calcium and/or potassium), crosses the cell membrane and pushes the electrical input past a threshold (action potential) necessary to stimulate nerve conduction and message transmission. Damage to nerve tissue in diabetic subjects as the result of microvascular changes affects the ability for these charged molecules to pass through the cell membrane thus changing the electrical input and output of nerve cells therefore changing the action potential threshold. This change results in dysfunctional nerve transmission as well as hyperexcitability of the nerve leading to nerve pain or altered sensation.
The Polyol Pathway²
Elevation of blood glucose levels above the normal range causes glucose to be shunted into the polyol pathway that converts glucose into sorbitol and then into fructose. During these conversions, the cofactors (substances that assist in the reaction) NADPH and NAD⁺ are heavily utilized and depleted. These cofactors are also required in the process of making and using glutathione, a powerful antioxidant necessary to combat the damage that free radicals can impose upon cellular structures and operations. Loss of these cofactors also leads to an increase in AGEs (advanced glycation end products) which result in the AGE-ing and accelerated death of tissues, specifically neuronal tissue in the case of diabetic neuropathy.
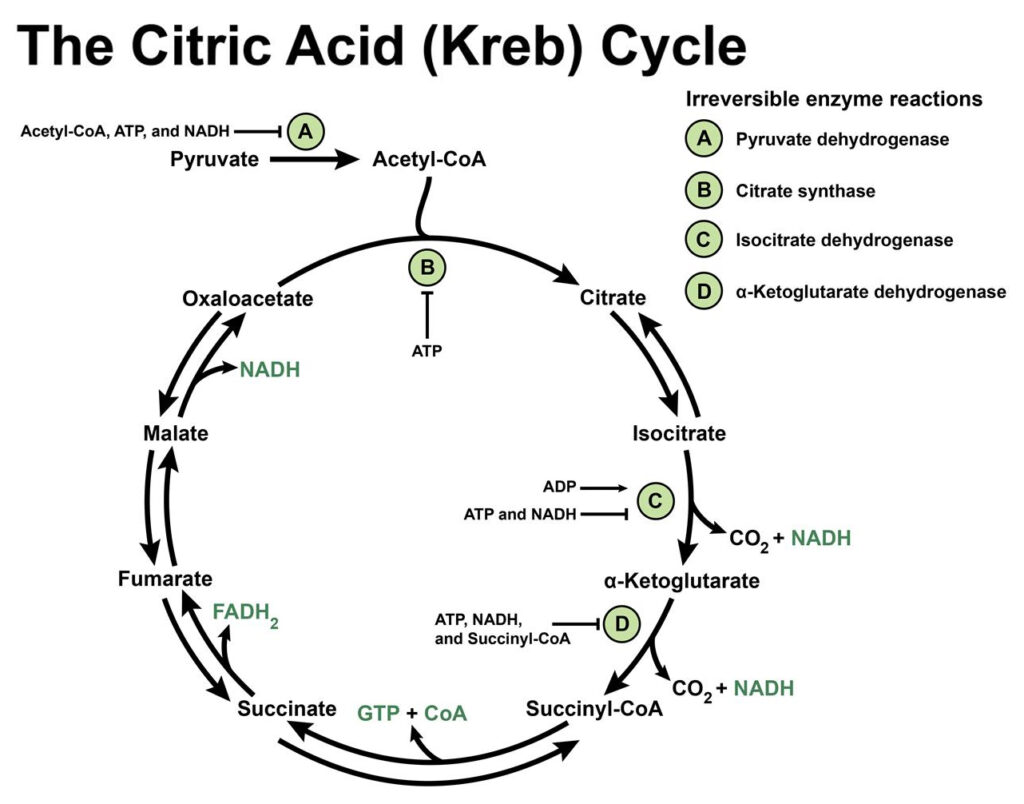
Hexosamine Pathway²·⁵
Much like the polyol pathway, excess blood glucose is redirected into the hexosamine pathway which breaks down glucose (glycolysis) to release ATP for energy, pyruvate to enter into the Kreb’s cycle to generate more energy and NADPH. Excess diversion of glucose into this pathway increases insulin resistance. Insulin resistance is an accessory finding in Type I diabetes and a precursor in those with Type II diabetes as well as obesity and metabolic syndrome, the latter of the two often found in conjunction in those diagnosed with diabetes. Upregulation of the Hexosamine pathway also results in the production of cell signaling molecules that promote an inflammatory state and/or cell death (apoptosis) which both significantly contribute to the development of diabetic neuropathy.
Nitric Oxide⁷
One of the ROS related to diabetic neuropathy is Nitric Oxide (NO) which is responsible for the regulation of blood flow, blood clotting and neuronal activity. NO is produced by the enzyme Nitric Oxide Synthase (NOS) that is found in three different forms that produce NO under different circumstances; endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS).
- Endothelial NOS lines the inner walls of blood vessels which is activated by the pulsation of blood flow and is responsible for the dilation of blood vessels to control blood perfusion into tissues. The NO created by eNOS activation is responsible for the development of new blood vessels (angiogenesis) in cases of wound healing and ulceration.
- Neuronal NOS aids in transmission and processing of information across synapses in the brain and from peripheral nerves to the brain.
- Inducible NOS is released in times of increased inflammation, i.e. bacterial infection or injury which results in a large production of NO. In pathological conditions, iNOS can be a very harmful enzyme implicated in cardiovascular disease, often found in those with long standing diabetes.
In states of elevated blood sugar, insulin is released, increasing the production of NO to counteract the effects of hyperglycemia. When blood glucose elevation becomes chronic, insulin resistance occurs and NO production from the enzymes eNOS and nNOS is decreased resulting in issues with blood flow and blood perfusion as well as nerve signal transmission between synapses which overtime can propagate the development of diabetic neuropathy.
Protein Kinase C (PKC)⁶
Protein kinase C, often found elevated in those with diabetes, is a group of enzymes that take part in many cellular functions, one of which is to assist in message signaling pathways. Elevations in PKC can alter the lining of the blood vessels which can increase the probability of cells leaching beyond the inner vessel wall as well as alter the vessel’s ability to dilate and constrict. Blood flow is also impacted by elevated PKC levels due to inhibition of the natural cycle of degeneration and regeneration of smooth muscle cells (SMCs) like those found in blood vessels and heart tissue. Nerve conduction and regeneration are both negatively impacted by the reduction in activity of an enzyme (Sodium-Potassium-ATPase) that controls the flow of sodium and potassium in and out of the cell in those with higher PKC levels as described above in relation to action potential.
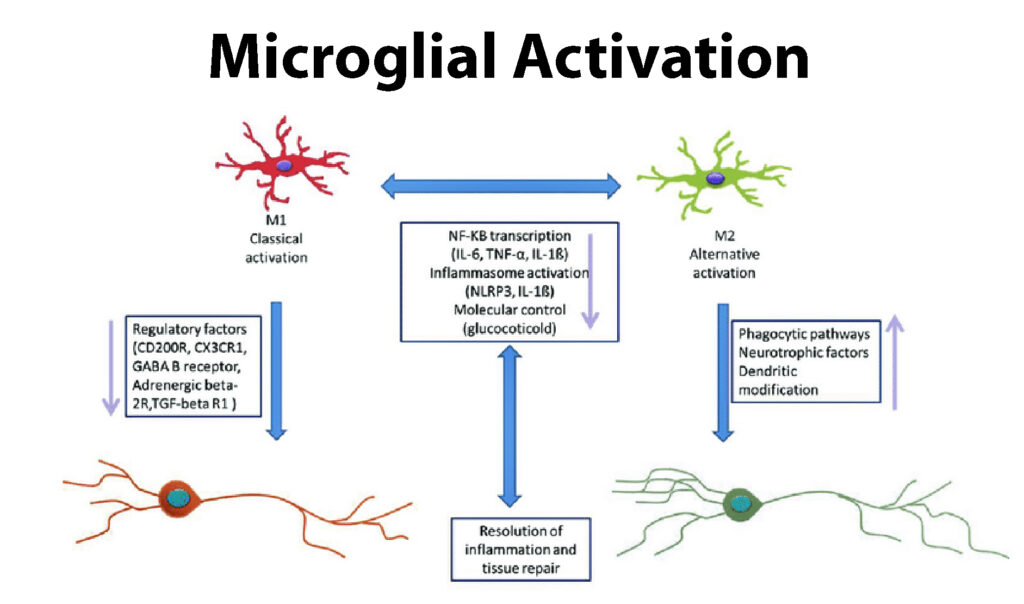
Microglial Activation²
Microglia are cells that reside within nervous tissue, participating in the formation of myelin and protection of nerve cells, among other functions. When damage to peripheral nerves occurs, there is a proliferation of microglial cells that activate inflammatory chemical messengers, upregulating a proinflammatory state within the nervous system. The activation of microglia in diabetes may also affect the action potential sodium channels creating a change in the influx of sodium into a cell thereby altering sensory perception in those with diabetes.
Central Sensitization²
Afferent nerve fibers that send messages from the body to the spinal cord and then up to the brain for processing are often altered in a diabetic state. These alterations result in hyperactivity of spinal sensory neurons leading to a heightened pain perception.
Brain Plasticity²
With increased input from peripheral nerves, a link has been made between the effects and functional changes in portions of the brain and spinal cord that perceive pain. Certain centers in the spinal column (spinothalamic tract) process harmful stimulation which is then sent to the receiving center of the brain (thalamus). Studies in diabetic rats have shown a hypersensitive response in spinothalamic neurons, resulting in enlarged receptive fields within the brain, elevated perception of harmful stimuli, and abnormal levels of spontaneous activity within the brain. This is believed to be in part due to plastic (structural) changes within the spinothalamic neurons.
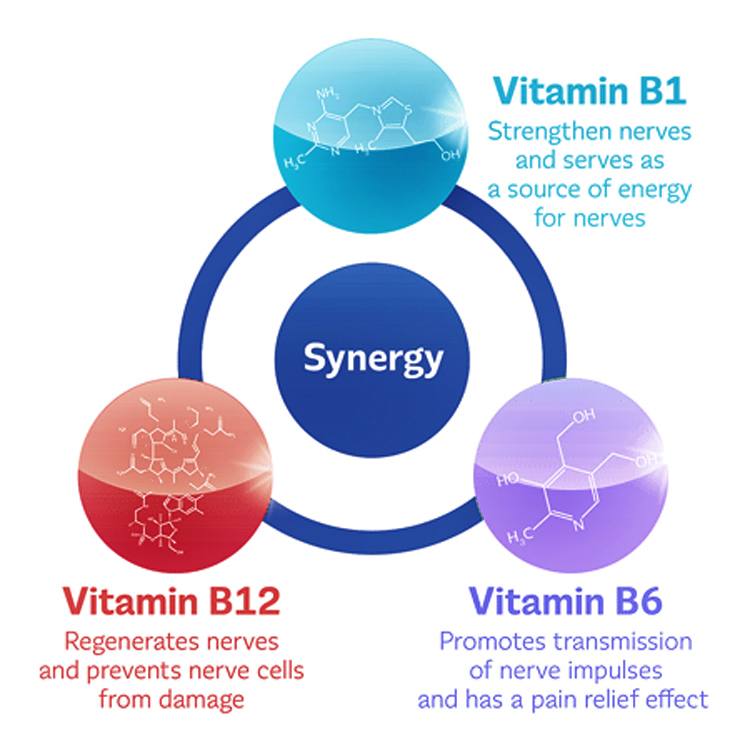
Low levels of B Vitamins ¹·⁸
These nutrients are imperative for the proper functioning of the central and peripheral nervous systems. Some diets can be lacking in these nutrients (i.e. vitamin B12 in vegetarian/vegan diets) whereas other people may be deficient due to pharmaceutical agents or lifestyle habits. A commonly overlooked contributor to the development of diabetic neuropathy is Vitamin B12 deficiency perpetuated by long term use of the blood sugar lowering drug Metformin. It has been shown that decreased absorption of Vitamin B12 can begin as soon as four months following the administration of Metformin. Within 5-10 years, individuals taking Metformin may see clinically overt symptoms of B12 deficiency.
The proposed mechanisms of B12 deficiency in those taking Metformin are due to:
1) Altered bowel motility causing an imbalance in the microbiome that manufacture B12,
2) inhibition and/or inactivation of B12 absorption,
3) Reduction of intrinsic factor necessary for B12 absorption, and
4) Reduction of calcium dependent absorption of B12 that can be mitigated with supplementation of calcium.
Lifestyle Factors
Several modifiable risk factors have been implicated in the initiation and progression of T2D, from diet and exercise to blood sugar management. For additional information, see BioSpec’s June 2020 Newsletter: Peripheral Neurology in Focus.
Please note that the partial list above has been condensed to focus on a limited number of factors. It is not a definitive list, as it covers a growing subject that is very multifactorial.
THERAPEUTIC APPROACHES
Mitochondrial and Antioxidant Support
Alpha Lipoic Acid: A therapeutic agent that has been well documented in research as an antioxidant with anti-inflammatory effects that has shown to reverse the symptoms of diabetic neuropathy caused by oxidative stress. R-Alpha Lipoic Acid is a better form that has demonstrated positive effects on blood sugar metabolism and nerve function.
N-Acetyl Cysteine (NAC): An immediate precursor to the powerful antioxidant glutathione that functions to reduce the effects of increased levels of reactive oxygen species and the downstream results of oxidative stress.
Acetyl L-Carnitine: An amino acid that plays a critical role in energy production from fatty acids as well as functions to increase activity of specific nerve cells within the central nervous system.
L-Selenomethionine: A precursor to the enzyme glutathione peroxidase that is essential for detoxification as well as a protectant against cellular oxidative damage.
Pyrroloquinoline Quinone (PQQ) and Coenzyme Q-10 (Ubiquinone): Antioxidants that work synergistically to enhance mitochondrial function.
Nicotinamide Adenine Dinucleotide (NADH): A critical electron donor in Complex I of the electron transport chain that further supports the production of CoQ10 in Complex II, driving the production of ATP in the mitochondrial membrane.
Silymarin Extract: Herbal support for liver detoxification to in turn reduce oxidative load on the system.
» Consider MITO-DETOX III, designed to address mitochondrial dysfunction through optimization of the electron transport chain and increased ATP synthesis.
Vitamin B12 Supplementation
Health practitioners will often order a serum B12 test to assess Vitamin B12 status. This test does not address the possibility of B12 not being properly utilized and has shown elevated levels in those with Vitamin B12 deficient neuropsychiatric symptoms. A better indicator of B12 status are the B12 metabolites, methylmalonic acid and homocysteine. An elevation in these would indicate a vitamin B12 deficiency. With B12 supplementation, methylcobalamin (activated B12) is the highly absorbable form.
» Consider METHYL B-12 2000, which contains 2,000 mcg (33,333% daily value) of Methylcobalamin along with 5-Methyltetrahydrofolate (5-MTHF) to reduce elevated homocysteine levels often found in those with diabetic neuropathy. This chewable tablet is an ideal option for those that have difficulty swallowing supplements.
Methylation Support
Addressing the methylation cycle is a critical approach to systemic inflammation, mitochondrial function, DNA preservation, detoxification and nerve health in those with diabetic neuropathy. This process is reliant upon adequate levels of vitamins B2, B6, Folate and B12 at various stages within the methylation pathway. Multiple studies have shown that specific forms of these B vitamins taken twice daily can help control and ease neuropathy symptoms.
» Consider METHYL-EASE HP, which fully supports proper methylation with high potency specific forms of Vitamin B2 as Riboflavin 5’-phosphate sodium, Vitamin B6 as pyridoxal 5’-phosphate, Folate in the form of both L-5-methyltetrahydrofolate and 5-formyltetrahydrofolate as well as B12 in the form of methylcobalamin.
Related Resources:
- Oxidative Stress VIDEO (Dr. Eric Berg)
- Enhance Mitochondrial Function VIDEO (Dr. Rudy Mueller)
- Peripheral Neuropathy Exercises PDF (OSU.edu)
- Neutraceuticals in focus: Diabetic Peripheral Neuropathy (BioSpec Newsletter, June 2020)
References:
- Cleveland Clinic. Neuropathy (Peripheral Neuropathy). Viewed 6 May 2020. https://my.clevelandclinic.org/health/diseases/14737-neuropathy
- Schreiber, A. K., Nones, C. F., Reis, R. C., Chichorro, J. G., & Cunha, J. M. Diabetic neuropathic pain: Physiopathology and treatment. World journal of diabetes. 2015; 6(3): 432–444.
- Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F , Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev. 2017; 2017: 8416763. Published online 2017 Jul 27.
- Lorenzi M. The Polyol Pathway as a Mechanism for Diabetic Retinopathy: Attractive, Elusive and Resilient. Exp Diabetes Res. 2007; 2007: 61038. Published online 2007 Jul 30. doi: 10.1155/2007/61038
- Buse MG. Hexosamines, insulin resistance and the complications of diabetes: current status. Am J Physiol Endocrinol Metab. 2006 Jan; 290(1): E1–E8.
- Geraldes P, King G.L. Activation of Protein Kinase C Isoforms & Its Impact on Diabetic Complications. Circ Res. April 30 2010; 106(8): 319-331.
- Burke T. Nitric oxide and its role in health and diabetes. Summary Overview. Personal Publication.
- Kibirige D, Mwebaze RJ. Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified? Diabetes Metab Disord. 2013; 12: 17. Published online 2013 May 7. doi: 10.1186/2251-6581-12-17
Medical Disclaimer: This content is for informational and educational purposes only. It is not intended to provide medical advice or to take the place of such advice or treatment from a personal physician. All readers/viewers of this content are advised to consult their doctors or qualified health professionals regarding specific health questions. Neither BioSpec Nutritionals, Practitioner Supply nor the publisher of this content takes responsibility for possible health consequences of any person or persons reading or following the information in this educational content. All viewers of this content, especially those taking prescription or over-the-counter medications, should consult their physicians before beginning any nutrition, supplement or lifestyle program.